OBJECTIVES
The Ultra-High Field MR group strives at providing state-of-the-art sequences and protocols, which take full advantage of the 7T system available at DRCMR. MR systems operating at fields of 7 tesla or above pose a series of technical challenges for reaching the full potential of the systems:
- The improved signal-to-noise at 7 tesla allows submillimeter structural and functional image resolution which offers new insights into the understanding of the organization and processes of the body in health and disease. However, a good compensation of subject motion is required to avoid image degradation, which would defeat the purpose of ultra-high resolution imaging. We work on fast image readout approaches and navigator-based correction methods to reduce the effects from motion and the increased physiological noise we unfortunately experience at higher field strength.
- The higher radiofrequency (RF) at 7 tesla causes strong interactions between the electromagnetic fields and the human body, which makes optimal RF control challenging within the allowed specific absorption rate (SAR) levels using traditional transmit approaches. Our solution is to develop more advanced excitation approaches and to use novel multi-transmit RF technologies available on our state-of-the-art equipment. These new transmit concepts will allow highly improved RF distributions in the human body, and thereby deliver superior image quality at safe SAR levels.
- Controlling motion and RF requires confinement of inhomogeneities and fluctuations in the main B0 field. We therefore work on advanced shim and dephasing techniques to not only achieve an ideal homogeneous field, but also to take advantage of being able to control the field temporally during the sequences. We exploit this to make novel zoom imaging methods, to exclude unwanted tissue such as fat as well as in advanced RF pulse designs.
RESEARCH PROJECTS
The technical innovations, made by the group, are available and applied to all clinical studies performed on the system. The group’s clinical interest ranges from high-resolution structural, functional and quantitative imaging to advanced spectroscopy editing and imaging. We apply these techniques to aging studies, studies of neurodegenerative diseases, in particular Parkinsonism, neuropsychiatric research and research on various other diseases. Examples of ongoing or upcoming projects typically conducted in synergy with other groups within or outside DRCMR are listed below:
- Diffusion weighted magnetic resonance spectroscopy at ultra-high field: Unravelling microstructural changes in cerebral white matter in patients with multiple sclerosis Henrik Lundell is currently pursuing the first clinical ultra-high field (7T) MR study in Denmark. In his project, he combines magnetic resonance spectroscopy with diffusion MRI to shed new light into the microstructural alterations in major motor white-matter tract caused by multiple sclerosis.
- Brain metabolite changes across the lifespan: a 7T MR study Anouk Marsman and Anna Lind Hansen exploit the high sensitivity of MRS at 7T to look into which role glutamate, GABA, GSH and lactate plays in neurochemical mechanisms that are important in brain development, function and plasticity as well as neuropsychiatric and neurodegenerative diseases.
- A generalized prospective motion correction framework for improved spectroscopy, structural and angiographic imaging Mads Andersen and Vincent Boer are developing techniques to update the position of the imaging/spectroscopy volume in real-time, as small head motion occur during scanning. Motion is monitored using extra sequence modules (navigators) that acquire dynamic MR data in pauses of the target imaging/spectroscopy sequence.
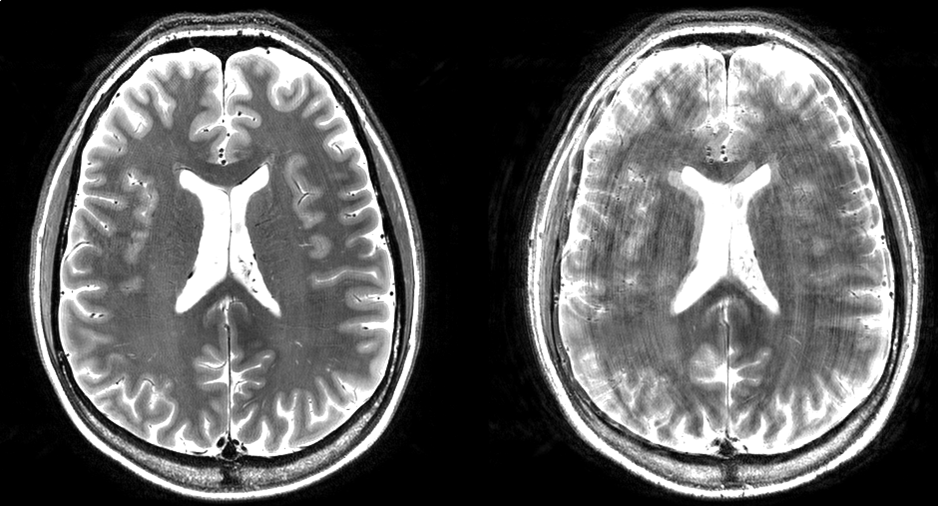
Figure 1: High resolution T2-weighted images acquired at 7T in a subject who moved during the scan. The left image was acquired with motion correction; the right image was acquired without motion correction. The motion was similar in timing and magnitude for the two acquisitions.









