The Neurophysics group is headed by Professor Axel Thielscher and situated both at DRCMR and DTU Health Tech. Our main focus is on advancing non-invasive transcranial brain stimulation (TBS) methods as a means to modulate and shape brain activity. Most TBS approaches use electric currents that are focally induced in superficial brain areas. We develop and apply biophysical models to reveal and optimize the current flow patterns in the brain and to estimate their impact on neural activity. The computational modeling work is complemented by applying neuroimaging approaches such as functional MRI (fMRI) and electroencephalography (EEG) to better characterize the impact of neurostimulation on brain activity. We are interested in human sensorimotor integration and motor control, which sets the neuroscientific scene in which we employ and test the NTBS methods.
The Neurophysics group contributes to a range of interdisciplinary project at DRCMR, such as the former BASICS project. We have tight interactions with the Control of Movement and the Brain Network Modulation groups (headed by Hartwig Siebner).
Funding sources
We would like to thank our funding sources.





Key projects
Simulation of Non-invasive Brain Stimulation (SimNIBS)
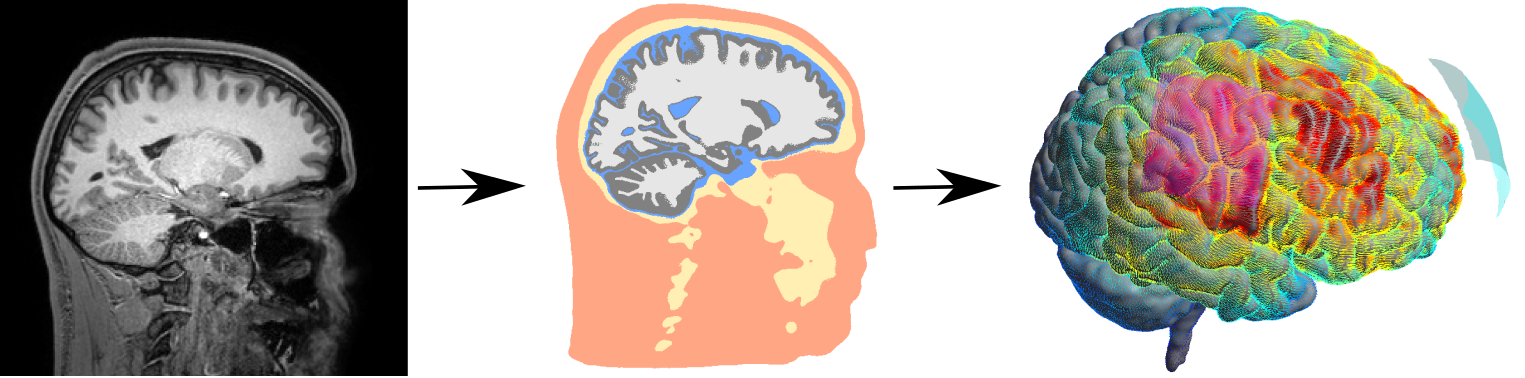
SimNIBS is both a scientific and an open-source software development project (Thielscher et al. 2011). We use field calculations to estimate the current flow generated by TBS methods in realistic head models, which are reconstructed from structural MR images. This allows us to determine the likely stimulated brain areas, and in turn to optimize the stimulation patterns. After developing the methodology, we are currently focusing on testing how well the estimated fields correlate with the experimentally observed stimulation effects. Simultaneously, we are strongly extending SimNIBS to include optimization methods for multi-electrode current steering for transcranial electric stimulation (TES) and to enable forward calculations for EEG in highly realistic head models. Oula Puonti is the main contributor to this project.
Automatic Whole-Head Segmentation
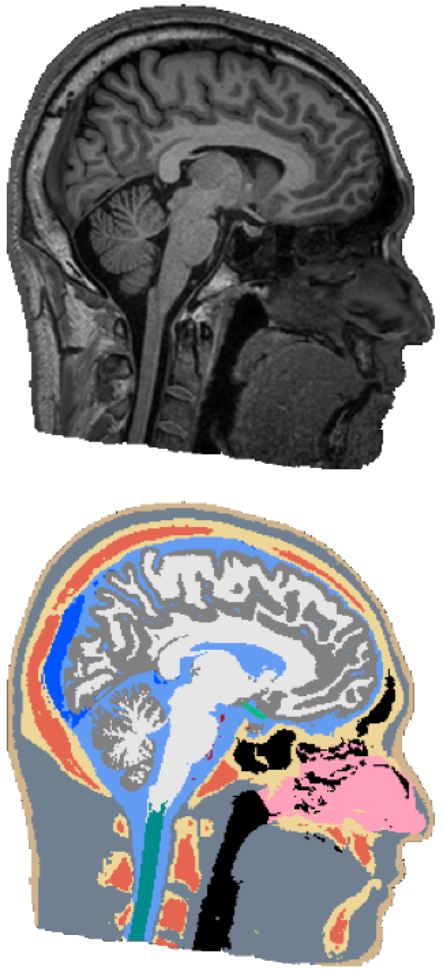
The electric fields induced by TBS are shaped largely by the individual anatomy of a target subject, and have complex and often counter-intuitive spatial distribution. In order to do targeted stimulation, or to locate sources in EEG or MEG, access to accurate models of the head anatomy is crucial. We are currently working on an automated segmentation approach for robust and accurate segmentation of 14 different head tissues from a subject’s MRI scan. The approach should generalize across different MRI scanners and scan-sequences, as well as work robustly on clinical MRI data, which often has a low resolution and movement-related artifacts. In the future, we are also planning to include automatic segmentation of pathologies, such as lesions or tumors, to facilitate the use of NIBS in clinical applications.Oula Puonti is the main contributor to this project.
Advanced Methods for MREIT and MRCDI
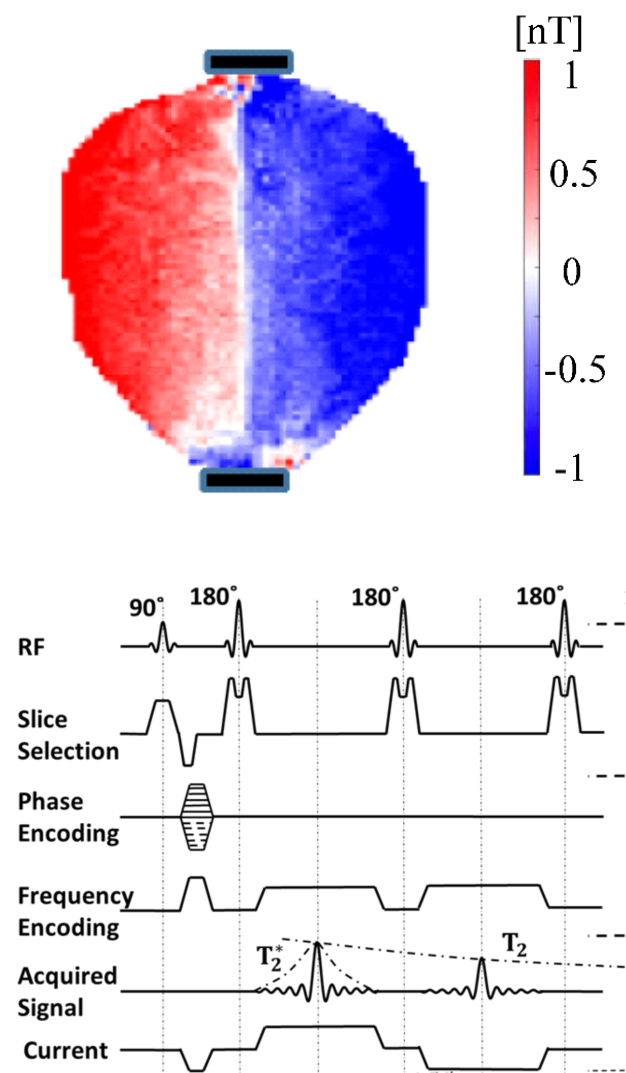 Magnetic resonance electrical impedance tomography (MREIT) and magnetic resonance current density imaging (MRCDI) are evolvling as methods to directly measure the ohmic conductivity distribution in the head and the current flow pattern generated by transcranial electric stimulation (TES). They would be tremendously useful to validate the field estimates from computational models, or might be used as alternative to them giving similar information. A main challenge for establishing MRCDI and MREIT in humans is the limited sensitivity of the MR measurements. We have demonstrated the so far highest sensitivity to injected currents worldwide (Göksu et al. 2018), and have shown reliable mappings of magnetic fields generated by injected currents as low as 1 milliampere. Currently, we are focusing on using the measurements to improve the SimNIBS forward simulations, and to mature the MR methods for use in larger studies. Cihan Göksu and Frodi Gregersen are main contributors to this project.
Magnetic resonance electrical impedance tomography (MREIT) and magnetic resonance current density imaging (MRCDI) are evolvling as methods to directly measure the ohmic conductivity distribution in the head and the current flow pattern generated by transcranial electric stimulation (TES). They would be tremendously useful to validate the field estimates from computational models, or might be used as alternative to them giving similar information. A main challenge for establishing MRCDI and MREIT in humans is the limited sensitivity of the MR measurements. We have demonstrated the so far highest sensitivity to injected currents worldwide (Göksu et al. 2018), and have shown reliable mappings of magnetic fields generated by injected currents as low as 1 milliampere. Currently, we are focusing on using the measurements to improve the SimNIBS forward simulations, and to mature the MR methods for use in larger studies. Cihan Göksu and Frodi Gregersen are main contributors to this project.
Weak Transcranial Focused Ultrasound Stimulation (TFUS) 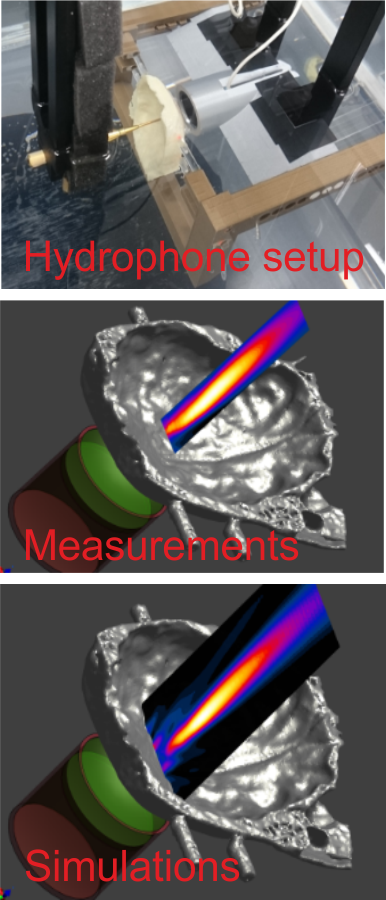
TFUS is emerging as new stimulation method with superior spatial resolution and the possibility to reach deeper areas of the brain. The existing data points towards a favorable safety profile of TFUS and suggests that it might be well usable in human applications (Pasquinelli et al. 2019). Computational dosimetry approaches will have a key role in future TFUS applications in order to ensure safe and neurally effective ultrasound intensities in the brain. However, dose control is made challenging by the strong impact of the heterogeneous skull on the TFUS beam, which can be difficult to simulate accurately. For this reason, we are working on the validation of simulations that are based on individual CT data of the skull. The simulations are based on the Sim4Life platform in a collaboration with the IT’IS foundation.
Characterizing the Effects and Side-effects of Transcranial Magnetic Stimulation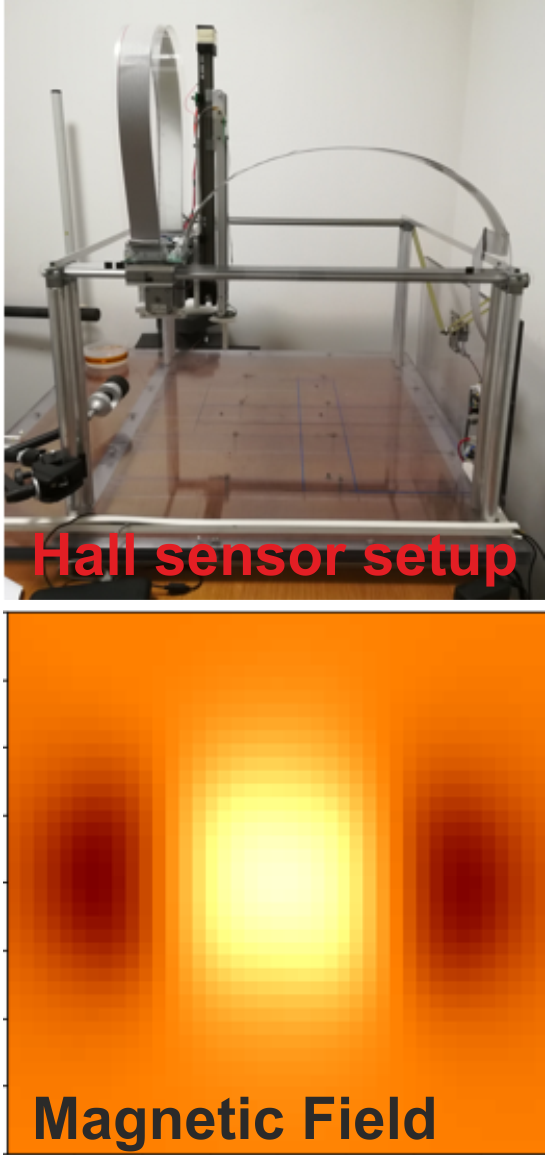
Transcranial magnetic stimulation (TMS) is a non-invasive method to stimulate cortical regions of the brain. It has established clinical and research applications, such as the treatment of depression. While TMS has been demonstrated to be effective, it causes also unwanted side-effects like pain and muscle twitches. We will develop models of existing commercial coils that will serve as an input to SimNIBS and extend our volume conductor models of the head in order estimate the stimulation of peripheral nerves in the skin. Our aim is to develop a quantitative computational model to estimate both the desired stimulation effect in the brain and the side-effects of TMS. We envision that this will open up new ways to develop more comfortable and accurate TMS coils. Maria Drakaki, Industrial PhD student at DTU and MagVenture, is the main contributor to this project.
Integrating TBS with Neuroimaging 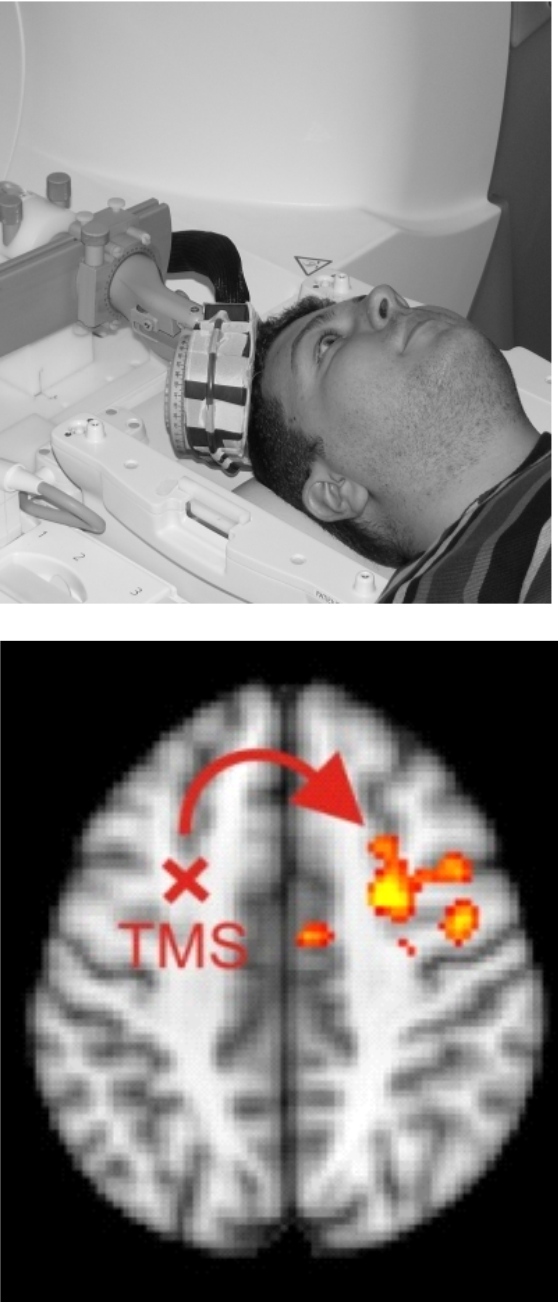
We are combining TBS with functional MRI (fMRI) to shed light on the stimulation effects at the brain network level and to characterize how the neural TBS effects depend on the physical TBS fields and the brain state. For example, we have shown that controlling the brain state by a behavioral task modulates the TBS effects, which could be used for optimizing TBS interventions (Moisa et al. 2012). Currently, in collaborations with the Brain Network Modulation group, we are integrating MR-based imaging of the neural TBS effects with SimNIBS calculations and MRCDI measurements of the induced fields. Our aim is to assess the usefulness of computational dosimetry approaches for predicting and optimizing physiological stimulation effects.
Novel methods for measuring neural magnetic fields 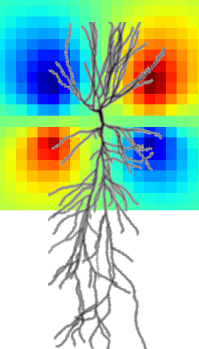
Nitrogen-vacancy centers in diamond are emerging as new sensors for highly sensitive magnetometry with superior spatial resolution and the ability to work at ambient temperatures. We are contributing to the Interdisciplinary Synergy Project “Interfacing emerging quantum technology with biology and neurophysiology” (BioQ) that is funded by Novo Nordisk Fonden and driven by our collaborators Ulrik Lund Andersen and Alexander Huck (DTU Physics). Extending our work in the EXMAD project, we aim to enable highly sensitive measurements of neural magnetic fields of neurons both in vitro and in humans.





